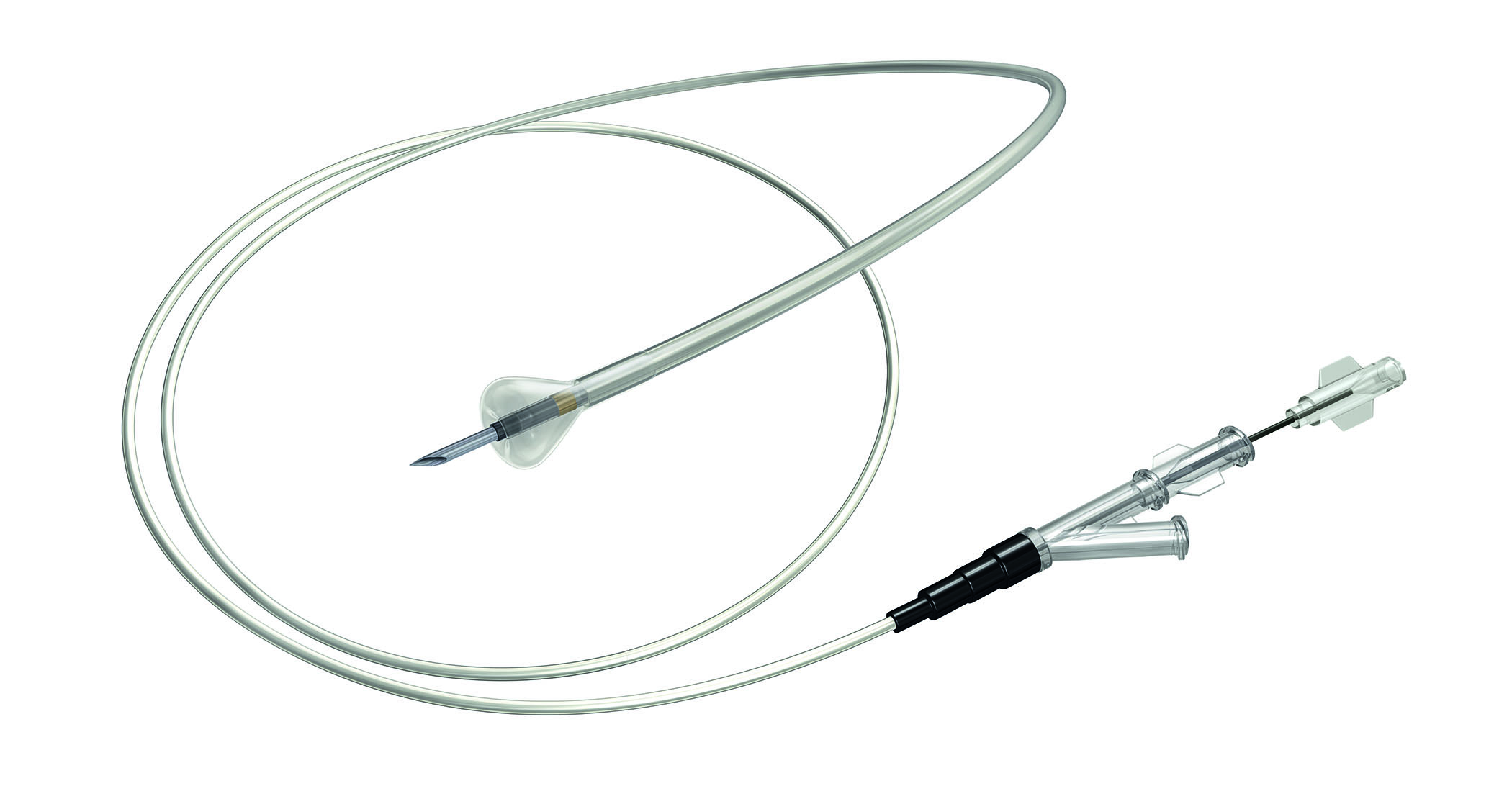
NATICK, Mass., Feb. 5, 2014 /PRNewswire/ -- Boston Scientific (NYSE: BSX) has announced the U.S. launch and first use of the OffRoad™ Re-Entry Catheter System, an important addition to the company's portfolio of tools to treat complete arterial blockages in the major arteries of the legs. These blockages, called chronic total occlusions (CTOs), are associated with advanced peripheral artery disease (PAD). The first use of the OffRoad System was performed by J.A. Mustapha, M.D., director of Cardiac Catheterization Laboratories, director of Endovascular Interventions, and director of Cardiovascular Research at Metro Health Hospital in Wyoming, Mich.
The OffRoad Re-Entry Catheter System is intended to help physicians navigate around complete arterial blockages by traveling within the tissue of the vessel wall (subintimal space). Once the catheter has passed the blockage, a unique conical-shaped positioning balloon is used to expand the subintimal space and direct a micro-catheter lancet to re-enter the vessel. This allows the physician to position a guidewire across the occlusion and to then treat the blockage using traditional endovascular techniques such as angioplasty and stenting.
"In my opinion, the biggest challenge with the subintimal approach is the ability of the device to re-enter the true vessel lumen after crossing," said Dr. Mustapha. "The unique design of the OffRoad System facilitates re-entry, giving me confidence that I will be able to successfully deploy the tools I need to treat the blockage. I look forward to adding OffRoad to my endovascular toolkit to address these challenging lesions."
Boston Scientific received U.S. Food and Drug Administration clearance in late 2013, following favorable results from the Re-ROUTE clinical trial. In the trial, investigators using the OffRoad System were successful in navigating around challenging CTOs in 84.8 percent of the enrolled patients, exceeding pre-specified trial goals.
"CTOs represent one of the most challenging forms of peripheral artery disease, putting patients at great risk of limb amputation and major cardiovascular events such as heart attack and stroke," said Jeff Mirviss, president, Peripheral Interventions, Boston Scientific. "Physicians rely on a variety of technologies and techniques to treat this condition, and with the addition of the OffRoad Re-Entry catheter system, we believe Boston Scientific is well-positioned to meet those needs with a complete portfolio of CTO solutions."
To view an animation of the OffRoad Re-Entry Catheter System in action, please click here.
About Peripheral Artery Disease
Peripheral artery disease is a circulatory disorder that results from a build-up of plaque in one or more of the arteries, most often in the legs. As the disease progresses, plaque accumulation may significantly reduce blood flow through the arteries, resulting in pain and increasing disability, with severe cases often leading to amputation of the affected limb. It is estimated that 12-14 percent of the general population is affected by PAD1.
- Shammas NW (2007). "Epidemiology, classification, and modifiable risk factors of peripheral arterial disease". Vasc Health Risk Manag 3 (2): 229–34. doi:10.2147/vhrm.2007.3.2.229. PMC 1994028. PMID 17580733
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 30 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding markets for our products, new product launches, product performance and importance, and competitive offerings. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Media: Ryan Davenport
763-494-2664 (office)
Global Media Relations
Boston Scientific Corporation
media@bsci.com
Investors: Susie Lisa, CFA
508-652-5345 (office)
Investor Relations
Boston Scientific Corporation
investor_relations@bsci.com
SOURCE Boston Scientific