MARLBOROUGH, Mass., April 16, 2015 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) is taking a new approach to evaluate the performance of the Vessix™ Renal Denervation System, initiating a study with a novel design to isolate the effects of the therapy in patients with high blood pressure, a silent cardiovascular killer affecting millions of people worldwide.
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7223453-boston-scientific-vessix-study
The first patient in the REDUCE-HTN: REINFORCE study was enrolled this week at Cardiology P.C. at Princeton Baptist Medical Center in Birmingham, Ala. by Farrell Mendelsohn, M.D., site principal investigator, and referred by Michael Wilensky, M.D. Boston Scientific received an investigational device exemption (IDE) for the study from the Food & Drug Administration (FDA) in December.
REDUCE-HTN: REINFORCE is a randomized, sham-controlled, multicenter study designed to isolate and demonstrate the effects of the Vessix™ Renal Denervation System by minimizing variability and factors that may have affected results in a competitive technology trial last year. Similar to pharmaceutical early effectiveness studies, patients in the study will undergo a four-week washout period prior to enrollment in which they will stop taking all hypertension medications.
"Patients with hypertension need a better therapy and we believe this study design will help us to understand better the clinical value of renal denervation," said Martin Leon, M.D., co-principal investigator and director, Center for Interventional Vascular Therapy at Columbia University Medical Center / New York-Presbyterian Hospital, New York City.
The REDUCE-HTN: REINFORCE study will enroll 100 patients. The primary efficacy assessment is the mean reduction in average 24-hour ambulatory systolic blood pressure (ASBP) at eight weeks post randomization. First results may be obtained in the first half of 2016.
"Previous results of renal denervation studies have been affected by a focus on patients with the difficult-to-define condition of treatment-resistant hypertension, made even more complex by uncertainties regarding their use of hypertension medications," said Michael Weber, M.D., co-principal investigator and professor of medicine, SUNY Downstate College of Medicine, Brooklyn, NY. "We need to find clarity, and we believe this innovative study design will enable us to do so."
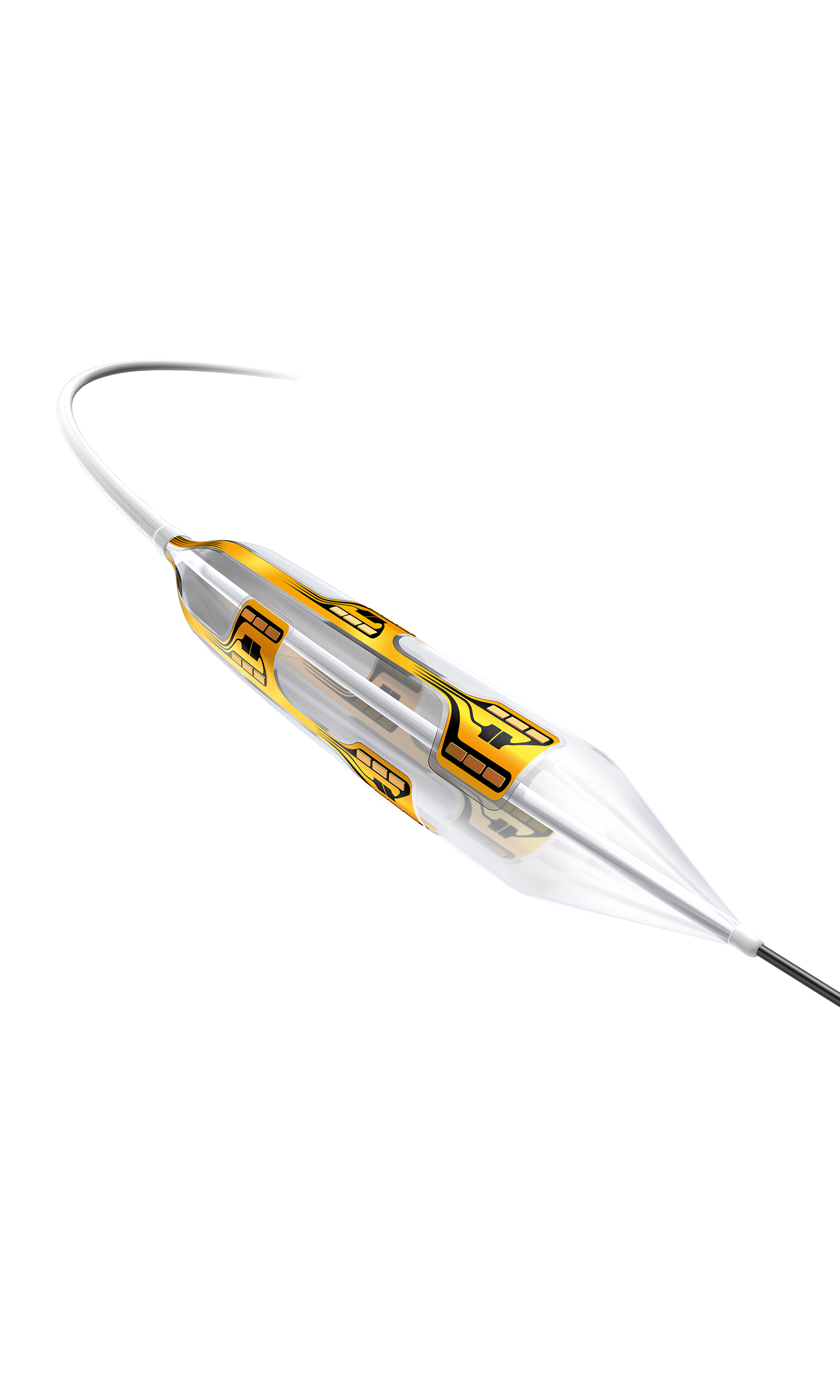
The Vessix System is a differentiated and advanced renal denervation system using a multi-electrode bipolar catheter designed to reduce procedural variability. It features a 30-second treatment time and an over-the-wire, balloon-based approach familiar to most cardiac and vascular specialists. The Vessix System has both CE Mark and Australian Government, Therapeutic Goods Administration (TGA) approval and is available for sale in Europe, the Middle East, Australia, New Zealand and select markets in Asia. The Vessix System is an investigational device and not available for sale in the United States.
"We believe the Vessix System is a meaningful innovation to help advance the care of patients with hypertension," said Jeff Mirviss, president, Peripheral Interventions, Boston Scientific. "That is why we worked with the broader hypertension clinical community to develop a unique and comprehensive study that will provide critical data to inform the field and demonstrate the true effect of renal denervation in reducing high blood pressure."
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 35 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding the REDUCE-HTN: REINFORCE study, regulatory approvals, clinical trials and impact of data, and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS
Media: Ryan Davenport
Global Media Relations
Boston Scientific Corporation
763-494-2664 (office)
media@bsci.com
Investors: Susie Lisa, CFA
Investor Relations
Boston Scientific Corporation
(508) 683-5565 (office)
investor_relations@bsci.com

SOURCE Boston Scientific Corporation