MARLBOROUGH, Mass., Sept. 29, 2015 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) announced today the launch of the Captivator™ EMR Device for Endoscopic Mucosal Resection (EMR), a minimally invasive alternative to an esophagectomy, a surgical procedure that removes part of the esophagus in upper gastrointestinal (GI) tract cases. EMR enables staging and removal of precancerous tissue and early esophageal cancer in the upper GI tract during an endoscopic outpatient procedure. The Captivator EMR Device is specifically designed for upper GI EMR and provides physicians with enhanced visualization, control and easy passage of devices.
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7223458-boston-scientific-captivator-emr-device/
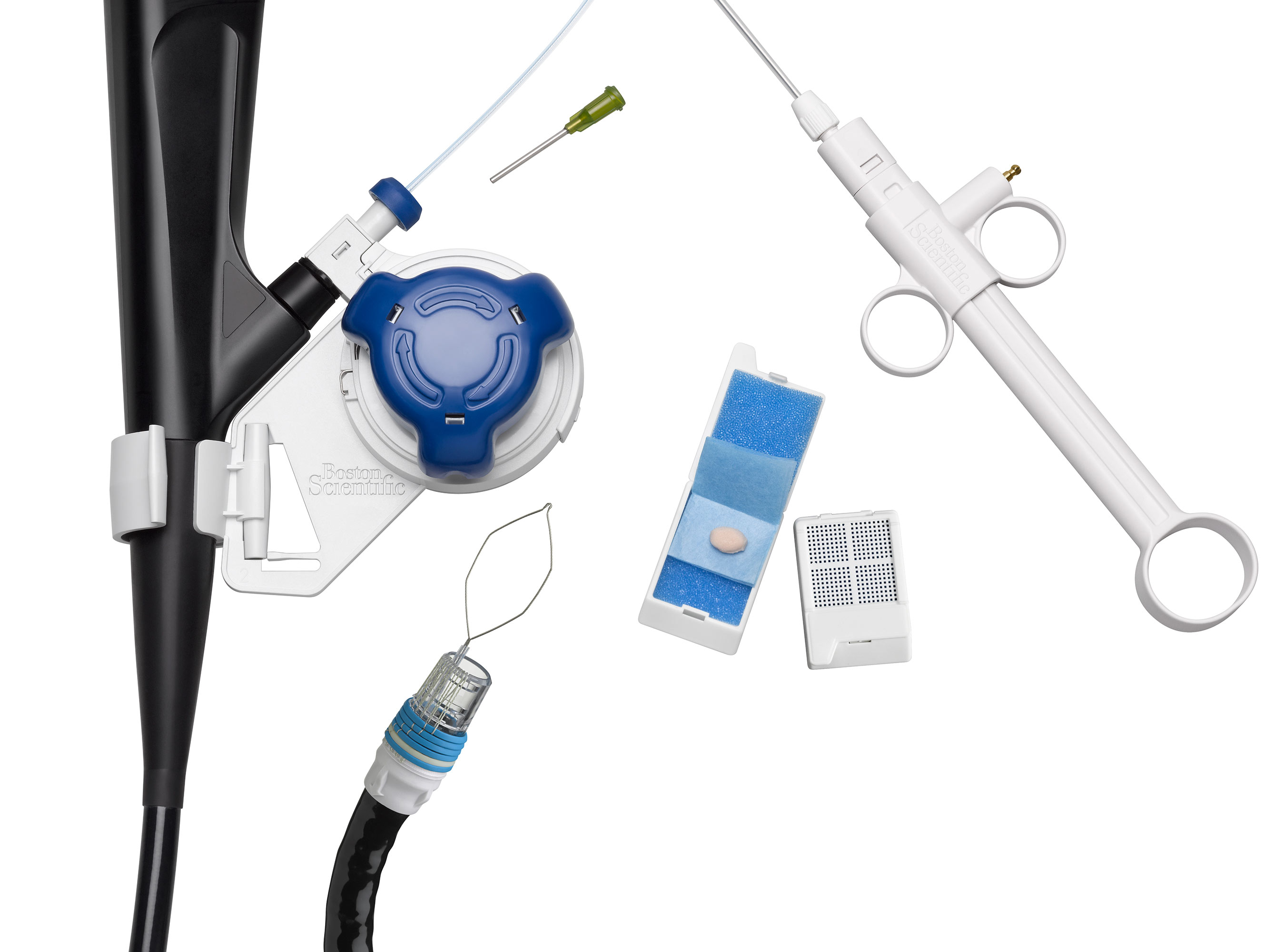
A common precursor to esophageal cancer is Barrett's Esophagus, which is a complication of acid reflux disease that causes abnormal changes to the lining of the esophagus. More than 900,000 people worldwide are diagnosed with Barrett's Esophagus[1]. Nearly 10 percent of patients with chronic heartburn develop Barrett's Esophagus and endoscopic management may help reduce the potential for an esophagectomy, a more invasive and costly surgery.[2],[3]
The company also enrolled the first patient in a multi-center, prospective, post-market registry initiated to study the performance of the Captivator EMR Device for endoscopic resection of abnormal tissue growth known as early neoplasia, specifically in Barrett's Esophagus. It will document procedural success, procedure time, quality of acquired tissue for histopatholgy, and procedural complications. The registry will enroll approximately 300 patients at 16 clinical centers in eight countries, and is expected to be completed in 2016.
"The Captivator EMR Device provides improved endoscopic visualization, so the resection can be better directed to the area of interest," said Dr. Jacques Bergman, professor of Gastrointestinal Endoscopy at University of Amsterdam, the Netherlands, and lead investigator of the global registry. "The enhanced view, along with easy passage of accessory devices and the ability to use compatible hemostatic devices to manage potential bleeding and complications may enable a safer endoscopic resection."
The Captivator EMR Device provides physicians with a 360 degree unobstructed peripheral view for more complete visualization of the diseased area when performing resections. And unlike other EMR devices, accessory devices such as the Boston Scientific Resolution™ Clip and Interject™ Needle can be used with the device during the procedure to manage potential complications quickly. The Captivator EMR Device is also currently the only EMR device that includes a pathology kit to provide added convenience for endoscopists and pathologists.
"As a leader in GI endoscopic devices, this launch complements our existing product offering for lower GI EMR procedures, and expands our portfolio into the growing upper GI EMR segment ," said David Pierce, senior vice president and president, Endoscopy, Boston Scientific. "The Captivator EMR Device provides physicians and patients with an important new option for staging and treating early esophageal neoplasia and cancer."
The Captivator EMR Device is available in the United States, Europe, Singapore, Australia and Puerto Rico.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 35 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding new product, markets for our products, product performance and impact and competitive offerings. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Media: Nisha Deo
408-893-9243 (cell)
Global Media Relations
Boston Scientific Corporation
Nisha.Deo@bsci.com
Investors: Susie Lisa, CFA
508-683-5565 (office)
Investor Relations
Boston Scientific Corporation
investor_relations@bsci.com
[1] American College of Gastroenterology, "New ACG Guideline: Diagnosis, Surveillance and Therapy of Barrett's Esophagus." November 2015 AJG.
[2] WebMD, "Barrett's Esophagus: Symptoms, Causes and Treatments."
[3] Datasource: Medicare's MedPar 2012 and Medicare's OPPS 2012 (Outpatient Prospective Payment System) files

SOURCE Boston Scientific Corporation