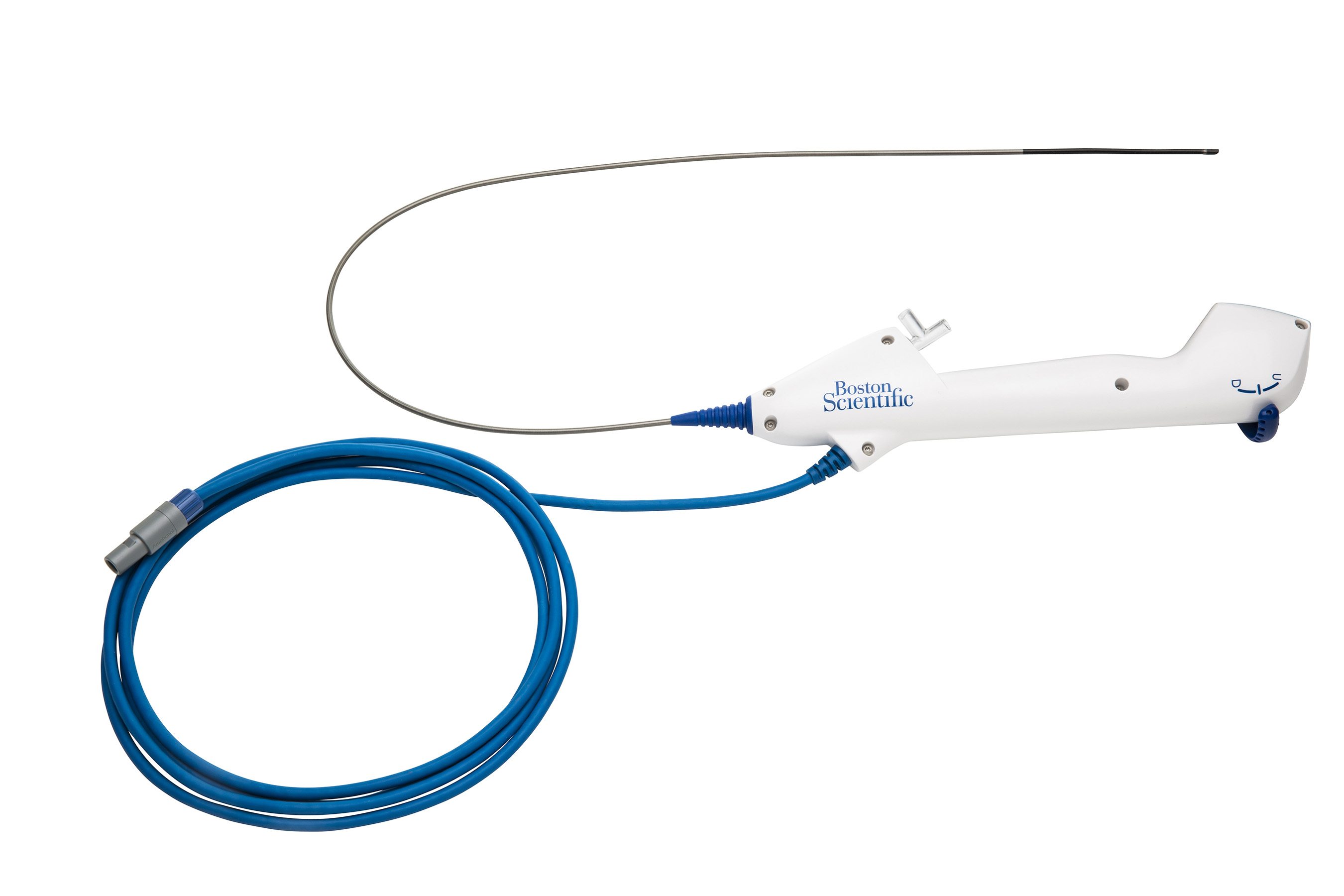
MARLBOROUGH, Mass., Jan. 12, 2016 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) today announced the U.S. and European launch of the LithoVue™ Single-Use Digital Flexible Ureteroscope for minimally invasive endoscopic procedures to diagnose and treat stones and other conditions of the kidney, ureter and bladder. By providing a single-use flexible ureteroscope for urologists, the LithoVue System is designed to address the inconsistent performance,1-4 operational challenges and costs associated with widely used reusable scopes that require maintenance, sterilization and reprocessing.1-7
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7223459-boston-scientific-lithovue/
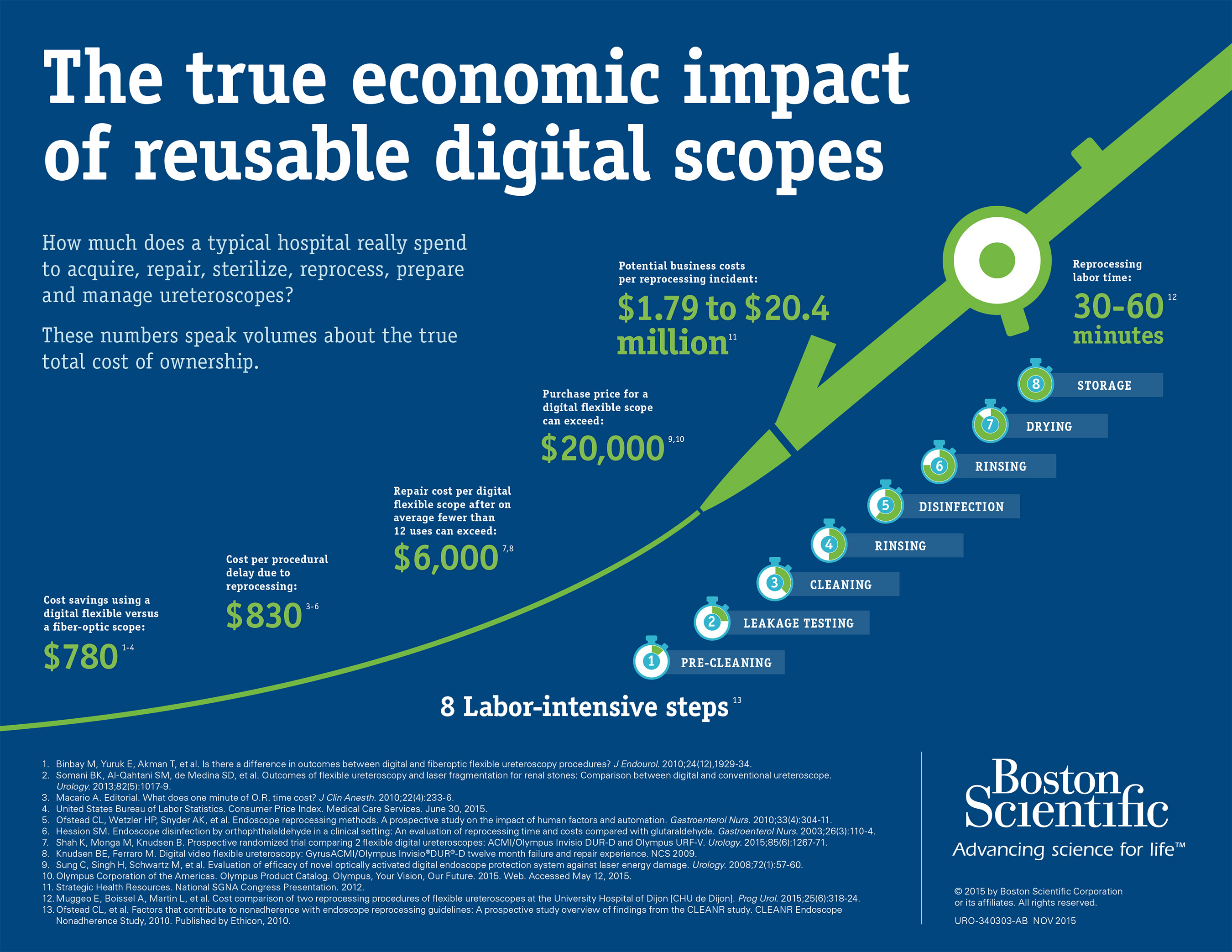
The LithoVue System is designed to deliver high-quality digital visualization and seamless navigation for consistent clinical performance during each patient procedure. Unlike reusable ureteroscopes, the LithoVue System can overcome the common challenges of unpredictable scope repairs and maintenance, reprocessing, sterilization and degradation of scope performance over time. The LithoVue System eliminates many of the inefficiencies and financial costs of ownership for reusable ureteroscopes, such as:
- On average, new digital flexible ureteroscopes require repair after fewer than 12 uses and cost more than $6,000 per repair.5,6
- Procedural delays are often due to reprocessing and repairs, and on average occur several times per day at a cost of $830 per delay.8-10
- Hospitals can eliminate many of the steps required to use, maintain and handle a reusable ureteroscope.11
"Flexible ureteroscopy is considered the gold standard for treating many stones in the ureter and kidney, and the LithoVue System provides high-quality visualization that is comparable to or better than the leading digital reusable ureteroscopes," said Glenn M. Preminger, M.D., Professor of Urologic Surgery and director of the Duke University Comprehensive Kidney Stone Center. "We believe that the LithoVue System offers a safe, effective and affordable solution that helps to avoid many of the hassles and unpredictable challenges of reusable ureteroscopes, without compromising visualization or maneuverability."
The LithoVue System features:
- High-resolution images: A digital CMOS imager in the 7.7F tip, with a working distance of 2mm–50mm, produces superb quality images across a depth of field that is equivalent to or better than those from other commonly used reusable scopes on the market.12
- Seamless navigation: Full 270 degree scope deflection in both directions provides accurate navigation toward the targeted treatment area.
- All-in-one solution: The LithoVue workstation monitor with integrated image processing software is mounted on a compact, rolling mobile cart which can be used alone during a ureteroscopic procedure or connected to existing monitors and integrated video systems in the operating room suite.
"At Boston Scientific, we are committed to providing innovative solutions to the healthcare challenges that our customers face every day," said Karen Prange, senior vice president and president, Urology and Pelvic Health, Boston Scientific. "The LithoVue System is an example of how we are focused on addressing unmet needs by providing urologists and hospitals a predictable, cost-effective, minimally invasive endoscope for the management of kidney stones."
The LithoVue System is now available in the U.S., Europe and New Zealand. For more product and important safety information, please visit: www.bostonscientific.com/lithovue. Or follow Boston Scientific Urology and Pelvic Health on Twitter at @bsc_urology.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 35 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our product launches and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; the closing and integration of acquisitions; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS
Media:
Tom Keppeler
508-683-6585 (office)
Media Relations
Boston Scientific Corporation
thomas.keppeler@bsci.com
Simonetta Balbi
+39 3387936422 (mobile)
+39 0106060281 (direct)
Media Relations – Europe
Boston Scientific Corporation
balbis@bsci.com
Investors:
Susie Lisa, CFA
508-683-5565 (office)
Investor Relations
Boston Scientific Corporation
investor_relations@bsci.com
- Mues AC, Knudsen BE. Evaluation of 24 holmium: YAG laser optical fibers for flexible ureteroscopy. J Urol. 2009;182: 348-54.
- Carey RI, Gomez CS, Maurici G, et al. Frequency of ureteroscope damage seen at a tertiary care center. J Urol. 2006;176:607-10.
- Collins JW, Keeley FX, Timoney A. Cost analysis of flexible ureterorenoscopy. Br J Urol. 2004;93(7):1023-6.
- Carey RI, Martin CJ, Knego JR. Prospective evaluation of refurbished flexible ureteroscope durability seen in a large public tertiary care center with multiple surgeons. Urology. 2014;84:42-5.
- Shah K, Monga M, Knudsen B. Prospective randomized trial comparing 2 flexible digital ureteroscopes: ACMI/Olympus Invisio DUR-D and Olympus URF-V. Urology. 2015;85(6):1267-71.
- Knudsen BE, Ferraro M. Digital video flexible ureteroscopy: GyrusACMI/Olympus Invisio®DUR®-D twelve month failure and repair experience. NCS 2009.
- Knudsen B, Miyaoka R, Shah K, et al. Durability of the next-generation flexible fiberoptic ureteroscopes: A randomized prospective multi-institutional clinical trial. Urology.2010;75:534-9.
- Macario A. Editorial. What does one minute of operating room time cost? J Clin Anesth. 2010;22:233-6.
- United States Bureau of Labor Statistics. Consumer Price Index. Medical Care Services. June 30, 2015.
- Hession SM. Endoscope disinfection by orthophthalaldehyde in a clinical setting: An evaluation of reprocessing time and costs compared with glutaraldehyde. Gastroenterol Nurs. 2003;26(3):110-4.
- Value Vantage. Day-in-the-Life Research, May 2014.
- Eisner B. Evaluating the image quality of a novel single-use digital flexible ureteroscope. J Endourol. 2015;29(1):A348.
SOURCE Boston Scientific Corporation