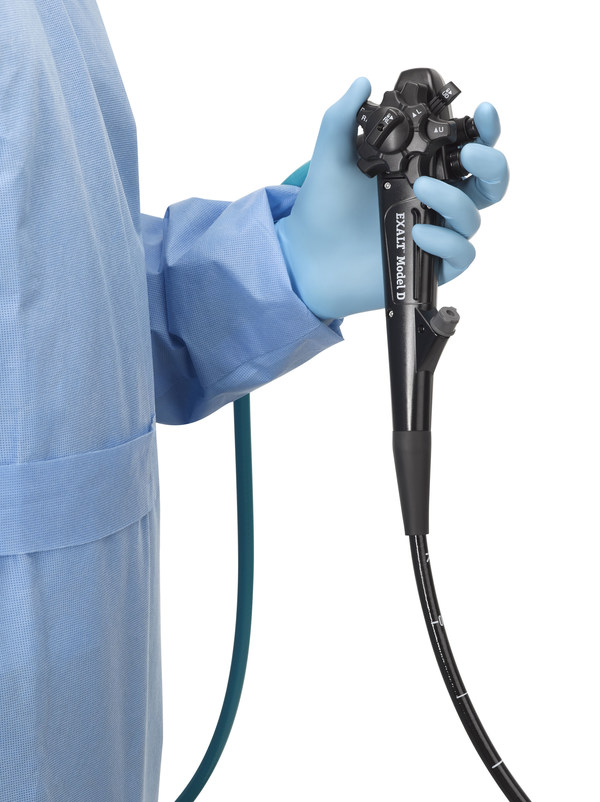
MARLBOROUGH, Mass., Dec. 13, 2019 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the EXALT™ Model D Single-Use Duodenoscope for use in endoscopic retrograde cholangiopancreatography (ERCP) procedures. The EXALT Model D Duodenoscope is the first and only FDA cleared single-use (disposable) duodenoscope on the market and was granted Breakthrough Device Designation from the FDA to ensure patients and healthcare providers have timely access to this device.

This groundbreaking device has been developed as an alternative to reusable duodenoscopes, eliminating the need for duodenoscope reprocessing and repairs, and allowing physicians to use a new, sterile device for every procedure. The EXALT Model D Duodenoscope builds on the familiar design of standard reusable duodenoscopes so that physicians experience a minimal learning curve when adopting this technology.
Every year, more than 1.5 million ERCPs are performed worldwide using duodenoscopes to diagnose and treat various pancreatic and biliary conditions.1,2
Reusable duodenoscopes are put through a rigorous disinfection process between uses in different patients and the vast majority of procedures with these devices are carried out safely and effectively; however, there have been a small number of cases in which infections have been transmitted between patients via contaminated devices, despite adherence to established protocols. As a result, the FDA has been working with duodenoscope manufacturers, medical societies, physicians and other stakeholders to address this concern. The FDA recently issued a recommendation that healthcare providers transition to duodenoscopes with disposable components or fully disposable devices, when they are available, and held an advisory committee meeting to discuss this process and other issues related to reducing infection transmission by reusable duodenoscopes.
"The EXALT Model D Duodenoscope was developed to support clinicians in their mission to deliver the highest quality patient care," said Art Butcher, senior vice president and president, Endoscopy, Boston Scientific. "As a leading industry partner committed to advancing the care of pancreaticobiliary diseases for over 30 years, helping to ensure the integrity of ERCP is inherent in our mission to advance science for life and transform patient lives. This device, which was granted Breakthrough Device Designation by the FDA, builds on our legacy of delivering meaningful innovation that improves clinical outcomes."
The FDA Breakthrough Devices Program is intended to help patients receive timely access to breakthrough technologies that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions.
Boston Scientific voluntarily conducted a consecutive case series of the EXALT Model D Duodenoscope and found that expert endoscopists were able to complete ERCPs across a wide range of complexity using the single-use duodenoscope.3
"With the EXALT Model D Duodenoscope, I can perform the same high-quality ERCP procedure with the added benefit of using a brand-new sterile device for each patient," said Dr. Raman Muthusamy, Medical Director of Endoscopy, Professor of Clinical Medicine, David Geffen School of Medicine at UCLA. "I believe the development of this device is a significant advancement in the evolution of endoscopy."
The company plans to commence a limited market release of the device in the U.S. during the first quarter of 2020.
Please visit www.bostonscientific.com/EXALT for more information about the EXALT Model D Duodenoscope.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for 40 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our product launches and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; the closing and integration of acquisitions; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS
Abhi Basu
Media Relations
(508) 683-4797 (Office)
Abhi.Basu@bsci.com
Susie Lisa, CFA
Investor Relations
508-683-5565 (office)
BSXInvestorRelations@bsci.com
- Internal Estimate
- United States Food and Drug Administration website: "Infections Associated with Reprocessed Duodenoscopes" August 29, 2019
- Muthusamy, V. Raman et al. Clinical evaluation of a single-use duodenoscope for endoscopic retrograde cholangiopancreatography. Clinical Gastroenterology and Hepatology, 2019, 0,0

SOURCE Boston Scientific Corporation