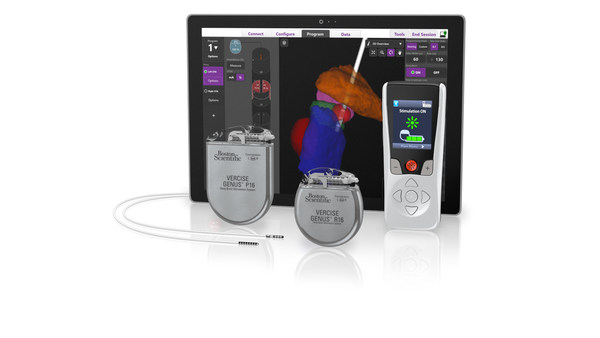
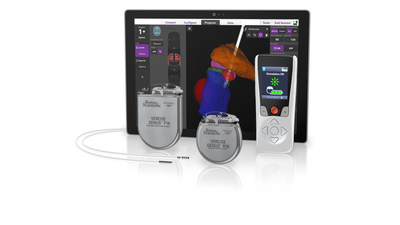
MARLBOROUGH, Mass., Sept. 9, 2020 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) announced it has received CE Mark and initiated a limited market release of the fourth generation Vercise Genus™ Deep Brain Stimulation (DBS) System in Europe. Featuring full-body MRI conditional1 and Bluetooth capabilities across the portfolio, the Vercise Genus System is indicated to treat the symptoms of Parkinson's disease (PD), essential tremor, and dystonia by delivering precisely targeted electrical stimulation in the brain designed to provide optimal symptom relief.
"With neurodegenerative movement disorders, the ability to deliver the right dose of stimulation where it is needed can make a remarkable difference in controlling an individual patient's symptoms," says Veerle Visser-Vandewalle, M.D., Ph.D., Cologne University Hospital, Germany.
Vercise Genus adds features for patients, including a low-profile two-in-one extension with the option of abdominal placement. New clinician software optimizes programming with integrated visualization using patient imaging via the company's exclusive relationship with Brainlab.
"Advanced directional stimulation combined with a visualization system based on a patient's own brain anatomy now provides the accurate navigation as well as the steering capability we need to optimize therapy outcomes," said Jens Volkmann, M.D., Ph.D., director and chairman, Department of Neurology at the University Hospital of Würzburg, Germany.
The fourth generation DBS system since 2012, Vercise Genus builds upon rapid, meaningful innovations such as multiple independent current control (MICC) and the Cartesia™ Directional Lead with integrated visualization. "For patients, the Vercise Genus DBS System continues the tradition of small, thin devices, and it provides Bluetooth programming which is important during times of social distancing," said Maulik Nanavaty, senior vice president and president, Neuromodulation, Boston Scientific. "Combined with the option of a 25-year rechargeable battery as well as the expanded MRI conditional1 feature available on our primary cell devices, patients can find the best option to suit their specific needs."
More than 10 million people worldwide are living with PD – a progressive, neurodegenerative disorder which causes stiffness, slowness and tremors due to the decrease of dopamine in the brain.2 Dystonia is the third most common movement disorder after essential tremor and PD, affecting more than half a million men, women, and children across Europe.3
In Europe, the Vercise Genus DBS System is indicated for use in unilateral or bilateral stimulation of the subthalamic nucleus or internal globus pallidus for treatment of levodopa-responsive PD which is not adequately controlled with medication and for treatment of intractable primary and secondary Dystonia, for persons 7 years of age and older. Thalamic stimulation using the Vercise Genus DBS System is indicated for the suppression of tremor not adequately controlled by medications in patients diagnosed with essential tremor or PD.
The Vercise Genus DBS System is not available for use or sale in the United States.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for 40 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our business plans and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Rainer Puster
Media Relations, Europe
+491754347057
Rainer.Puster@bsci.com
Rochelle Silsbee
Media Relations, U.S.
+1 (818) 812-7320 (office)
Rochelle.Silsbee@bsci.com
Susie Lisa, CFA
Investor Relations
+1 (508) 683-5565 (office)
BSXInvestorRelations@bsci.com
1 1.5 Tesla MRI conditional when all conditions of use are met
2 https://www.epda.eu.com/about-parkinsons/what-is-parkinsons/
3 https://dystonia-europe.org/about-dystonia/dystonia
SOURCE Boston Scientific Corporation