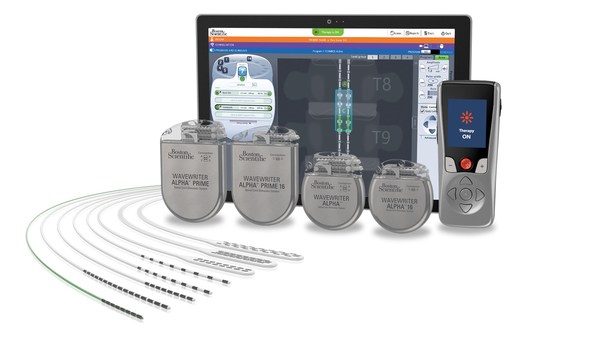
MARLBOROUGH, Mass., Sept. 30, 2020 /PRNewswire/ -- Boston Scientific (NYSE: BSX) today announced the European launch of the WaveWriter Alpha™ portfolio of Spinal Cord Stimulator (SCS) Systems. The portfolio, consisting of four MRI conditionali, Bluetooth-enabled implantable pulse generators (IPGs), offers expanded personalization based on patient needs, including rechargeable and non-rechargeable options, and access to waveforms that can cover multiple areas of pain.
Chronic pain, defined as continuous and long-term pain lasting more than 12 weeks, impacts approximately 100 million people across Europe.ii,iii SCS therapies provide pain relief by delivering pulses of mild electric current to the spinal cord to interrupt pain signals traveling to the brain. The WaveWriter Alpha SCS Systems received CE Mark and are indicated as an aid in the management of chronic intractable pain. It is also indicated for peripheral nerve stimulation of the trunk for pain management.
The systems feature combination therapy, the only SCS portfolio that has the ability to layer paraesthesia and paraesthesia-free options simultaneously, and support up to 32 contacts that target specific nerves along the spinal cord to meet the personal pain relief coverage needs of the individual patient. The Bluetooth platform enables faster programming that can be done while maintaining a typical physical distance of 10 feet (3meters) between the programmer and the patient.
"Being able to offer my patients different therapy options is important because it provides them with pain relief that can maintain long-term results," said Jan Vesper, M.D. Ph.D., Department of Functional Neurosurgery and Stereotaxy at the University Hospital of Düsseldorf, Germany. "The combination of different therapy features that promote simplicity like the WaveWriter Alpha SCS Systems enable enhanced personalization in the advanced treatment of chronic pain."
The COMBO randomized control trial, which compared the effectiveness of SCS with multiple modalities to conventional SCS in patients with chronic pain, found 88% of patients were responders with multiple modalities, which is defined as patients achieving 50% or greater pain relief compared to baseline. Patients also realized a significant 26-point improvement in functional disabilityiv where many patients who were "severely" or "moderately disabled" were able to return to many of their daily activities.v Multiple Level 1 RCTs and real-world studies support the design of Boston Scientific's SCS therapy.vi,vii,viii,ix,x
"Chronic pain can severely impact a patient's ability to carry out daily activities," said Jan Willem Kallewaard, M.D., Rijnstate Hospital, Arnhem, The Netherlands. "With the WaveWriter Alpha SCS Systems, not only are patients likely to achieve a successful outcome, but their quality of life is also being dramatically improved by regaining many functional abilities."
"The launch of the WaveWriter Alpha SCS Systems in Europe represents a significant step forward in the treatment of chronic pain," said Maulik Nanavaty, senior vice president and president, Neuromodulation, Boston Scientific. "By unifying our portfolio of SCS devices and offering the latest therapy advances, we remain committed to advancing meaningful innovation, providing both physicians and patients access to the full benefits of our entire portfolio of devices."
The WaveWriter Alpha SCS Systems are not available for use or sale in the United States.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for 40 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our business plans and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Rainer Puster
Media Relations, Europe
+491754347057
Rainer.Puster@bsci.com
Rochelle Silsbee
Media Relations, U.S.
+1 (818) 812-7320 (office)
Rochelle.Silsbee@bsci.com
Susie Lisa, CFA
Investor Relations
+1 (508) 683-5565 (office)
BSXInvestorRelations@bsci.com
____________________
i The WaveWriter Alpha™ and WaveWriter Alpha™ Prime Spinal Cord Stimulator Systems provide safe access to full-body 1.5T MRI scans when used with specific components and exposed to the MRI environment under the defined conditions in the ImageReady™ MRI Full Body Guidelines for WaveWriter Alpha™ and WaveWriter Alpha™ Prime Spinal Cord Stimulator Systems
ii Mills S et al. Identification and Management of Chronic Pain in Primary Care: A Review of Current Psychiatry Reports. 2016.
iii Policy Connect. About Chronic Pain. https://www.policyconnect.org.uk/cppc/about-chronic-pain Accessed March 2012
iv Oswestry Disability Index
v Wallace M et al. Outcomes of a Prospective Randomized Controlled Trial Utilizing a Spinal Cord System Capable of Multiple Neurostimulative Modalities (COMBO). NANS Annual Meeting. January 2020.
vi Thomson, Simon et al. Effects of Rate on Analgesia in Kilohertz Frequency Spinal Cord Stimulation: Results of the PROCO Randomized Controlled Trial. Neuromodulation, January 2018.
vii North J et al. Outcomes of a Multicenter, Prospective, Crossover, Randomized Controlled Trial Evaluating Subperception Spinal Cord Stimulation at ≤1.2 kHz in Previously Implanted Subjects. Neuromodulation. January 2020.
viii Metzger C et al. Pain Relief Outcomes Using an SCS Device Capable of Delivering Combination Therapy with Advanced Waveforms and Field Shapes. Expert Rev Med Devices. September 2020.
ix Veizi E et al. Spinal Cord Stimulation (SCS) with Anatomically Guided (3D) Neural Targeting Shows Superior Chronic Axial Low Back Pain Relief Compared to Traditional SCS-LUMINA Study. Pain Med. November 2018.
x Paz et al. HALO Study: Exploration of High and Low Frequency Outcomes in Sub-p (n=30), INS 2019
SOURCE Boston Scientific Corporation