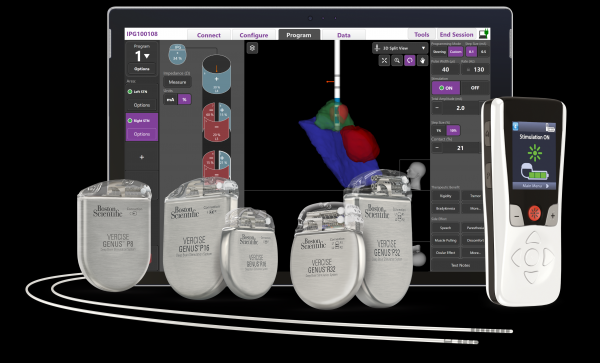
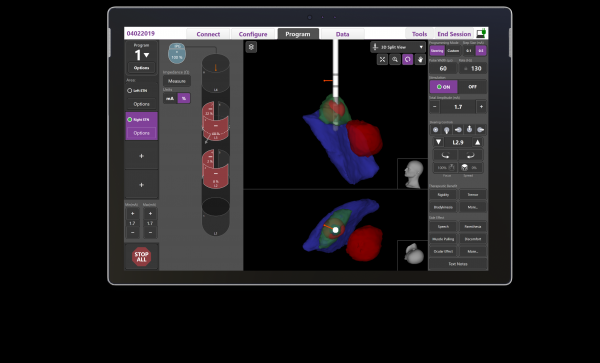
MARLBOROUGH, Mass., April 12, 2022 – Boston Scientific Corporation (NYSE: BSX) today announced it has received U.S. Food and Drug Administration (FDA) approval for its latest image guided programming software, Vercise™ Neural Navigator with STIMVIEW™ XT. Developed in collaboration with Brainlab AG, a leading software-driven medical technology company, STIMVIEW XT enables clinicians in real-time, the ability to visualize both lead placement and stimulation modeling of the brain anatomy of their patients living with Parkinson’s disease or essential tremor.
STIMVIEW XT used with the Vercise Genus™ Deep Brain Stimulation[i] (DBS) portfolio, is engineered to be the most advanced and integrated visualization software for DBS programming. The software enables patient-specific 3D visualization of the anatomy for clinicians to better personalize therapy to meet each patient’s needs. It seamlessly integrates into the Vercise Genus programming interface, designed to help localize lead placement, reduce programming time and enable more informed treatment decisions.
“It’s exciting that clinicians will now have access to more sophisticated image guided programming tools supporting personalized DBS therapy,” said Jill Ostrem, M.D., medical director, University of California San Francisco, Movement Disorders and Neuromodulation Center. “This advancement with STIMVIEW XT may also save time for the clinician as it could help avoid the trial and error in finding the precise location. Prolonged periods of time in adjusting stimulation settings can be stressful and tiring for patients.”
In a recent study, clinicians were able to adjust patient stimulation in an average of 20 minutes[ii] with the Boston Scientific visualization software -- lowering programming time by 56 percent.[iii]
“Every person’s experience living with Parkinson’s disease or essential tremor is unique, and their treatment should be as unique,” said Maulik Nanavaty, senior vice president, Neuromodulation, Boston Scientific. “Our technologies enable clinicians to precisely see, shape, and steer DBS therapy to meet their patients’ individual needs. This latest advancement is a testament to how we’ll continue to deliver on meaningful innovations that support doctors.”
The U.S. launch of Vercise Neural Navigator with STIMVIEW XT follows the European launch of the software providing broader access to clinicians who treat people living with Parkinson’s disease or essential tremor.
For more information, visit STIMVIEWXT.com.
About the Boston Scientific DBS System
The fourth-generation Vercise Genus™ Deep Brain Stimulation (DBS) system is designed to treat the symptoms of Parkinson’s disease and essential tremor by delivering targeted electrical stimulation via surgically-implanted leads in the brain connected to an implantable pulse generators (IPG). The portfolio, approved for conditional use in a magnetic resonance imaging (MRI) environment,1 consists of a family of Bluetooth-enabled, rechargeable and non-rechargeable, IPGs that power Cartesia™ Directional Leads, designed to provide optimal symptom relief.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 40 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like “anticipate,” “expect,” “project,” “believe,” “plan,” “may”, “estimate,” “intend” and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our business plans and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future U.S. and global economic, political, competitive, reimbursement and regulatory conditions; new product introductions; expected procedural volumes; the closing and integration of acquisitions; demographic trends; intellectual property rights; litigation; financial market conditions; the execution and effect of our business strategy, including our cost-savings and growth initiatives; and future business decisions made by us and our competitors. New risks and uncertainties may arise from time to time and are difficult to predict, including those that have emerged or have increased in significance or likelihood as a result of the COVID-19 pandemic. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Media:
Jessica Sachariason
+1.415.730.2310
Investor Relations:
Lauren Tengler
+1.508.683.4479
lauren.tengler@bsci.com
[i] The Vercise Genus™ DBS System provides safe access to full-body 1.5T MRI scans when used with specific components and the patient is exposed to the MRI environment under specific conditions defined in the supplemental manual ImageReady™ MRI Guidelines for Boston Scientific DBS Systems.
[ii] Lange et al. Reduced programming time and strong symptom control even in chronic course through imaging-based DBS Programming. Frontiers in Neurology. 2021;12. doi:10.3389/fneur.2021.785529
[iii] Compared with standard clinical based programming, p=0.039